Bíró Szabolcs állításából ezt szűrheti le az olvasó:
Olyan készítményeket érdemes keresni, amelyekben a B5-vitamin pantetin formájában van, ami a B5-vitamin jobb/aktívabb formája, mint a pantoténsav (pl.: Ca-pantotenát).
Bíró Szabolcs indoklása:
A Pantetin panteteinre bomlik, ami sejten belül már több lépéssel közelebb van a végső, aktív Koenzim-A (CoA) formához, mint a pantoténsav/pantotein, így az könnyebben alakul CoA-vá. Ennek igazolására 1 db szakcikket meg is ad referenciának, azonban abban csak megemlítik a pantetint, mint ami esetleg hatásos lehet azoknál, akik a sima B5-vitamint (pantoténsavat) nem tudják átalakítani (7 évvel később egy vizsgálat rácáfol erre, de már előtte lévő vizsgálatok is indirekt rácáfoltak).
Az én állításom:
A pantetin, egy élelmiszereinkben elő nem forduló B5-vitamin analóg, amely 10x drágább, de maximum azonosan jó, mint a sima pantoténsav/pantotenát (pl.: Ca-pantotenát), mivel szájon át szedve eleve 100%-osan átalakul pantoténsavvá, mielőtt a véráramba jutna, a hasznosulása pedig kevésbé jó, mint a pantoténsavnak (pl.: Ca-pantotenátnak). Farmakológiai/szuprafiziológiás (óriási) dózisokban alkalmazva a pantetin rendelkezik olyan hatásokkal, amelyekkel a B5-vitamin nem rendelkezik semmilyen dózisában. A pantetin ezen hatásai azonban nem a B5-vitaminná való alakulásának képessége miatt, vagy a B5-ből képződő aktív koenzim (CoA) szintet emelő képességéből adódnak, azaz nem abból, hogy „aktívabb” vagy jobban hasznosuló B5-vitamin forma lenne, hanem abból, hogy ciszteaminná bomlik a szervezetben. B5-vitaminként a pantetin használata semmiképpen sem előnyös a mezei pantoténsavhoz képest. A pantetinnek tehát van ugyan a B5-vitamintól eltérő terápiás hatása szuprafiziológiás/farmakológiai dózisokban alkalmazva, azonban B5-vitaminként való használatának maximum nanokolloid rendszer részeként, vagy esetleg beinjekciózva lenne csak értelme, óriási dózisban adagolva, ugyanis szájon át szedve előbb alakul át sima pantoténsavvá a vékonybélben, minthogy a véráramba jutna. A pantetin használata mellékhatásmentes ciszteamin forrásként sok esetben jó hatású (pl.: koleszterin csökkentés céljából), de B5-vitamin forrásként nincs helye készítményekben. Nem baj, ha van bennük, de csak fölöslegesen drágítja őket, így csupán marketing szerepe van.
Az én indoklásom:
Élelmiszerekben a B5-vitamin főleg Koenzim-A (CoA) és 4-foszfopantetein formájában van jelen, és csak kis részben pantetein és pantoténsav formájában. [1,3,5,6] A CoA a már kész, „legaktívabb” formája a B5-nek, azaz ő maga a koenzim, a végső forma, ami a B5-ből alakul a sejtjeinkben. A 4-foszfopantetin pedig 2 lépéssel a CoA előtt lévő formája. Hasonlóan tehát a B1-, B2- és B3-vitaminhoz, élelmiszereinkből a B5-vitamint is már főleg abban a formában vesszük magunkhoz, amivé később a sejtjeinkben alakulnia kell, azaz a kész koenzim formájában. Ez természetes, hiszen amit elfogyasztunk, az is élőlény, és azért vannak bennük ezek a vitaminok, mert nekik kell (az ő sejtjeiknek), nem nekünk termelik őket, az ő sejtjeik is így, a kész formáikban használják őket. Mi azonban nem tudjuk ezeket hasznosítani közvetlenül, nem kerülnek csak úgy be az elfogyasztott élelmiszer sejtjeiből a mi sejtjeinkbe. Először az emésztőrendszerünkben szét kell bontanunk őket a legegyszerűbb formáikra, hogy a bélsejtjeinken keresztül átjuthassanak a véráramunkba, majd szisztémásan a sejtjeinkhez a vérárammal eljussanak, és a sejtjeinkbe bekerülhessenek. A B1 esetében ez a felszívódni/hasznosulni/sejtekbe bekerülni képes forma a tiamin, a B2 esetében a riboflavin, a B3 esetében a niacin/niacinamid, míg a B5-vitamin esetében ez a pantoténsav/pantotenát. Először tehát lebontjuk a már kész formákat a legkezdetlegesebb/legegyszerűbb/legkisebb formára, hogy eljusson és bejusson a sejtjeinkbe, amikben aztán újra felépül a legegyszerűbb formából a kész forma. Az alábbi ábra mutatja ezt jól a B5-vitamin esetében.[1] (A piros nyilak az élelmiszerekből származó kész formák lebontásának útját mutatják pantoténsavvá/pantotenáttá, míg a zöld nyilak a kész koenzim forma sejten belüli felépítésnek lépéseit pantoténsavból).
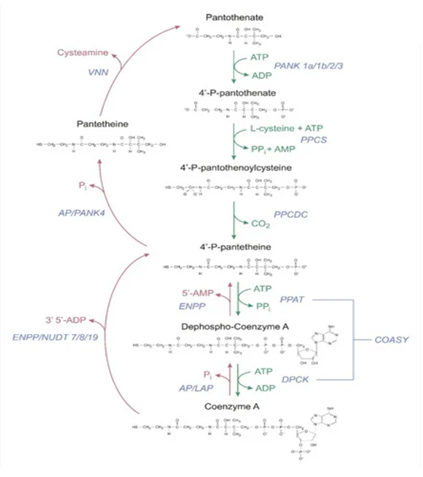
Étrendkiegészítőkben a B5-vitamint nátrium- vagy kalcium-pantotenát formájában szokták alkalmazni, amik lenyelve vagy bármiben feloldva azonnal nátriumra illetve kalciumra és pantoténsavra bomlanak (disszociálnak). A dexpantenol egy másik forma, de annak inkább krémekben van helye, így arra nem térek ki. A pantetin pedig egy újabban engedélyezett formája, amely a B5-vitaminnak egy tulajdonképpen mesterséges analógja, ugyanis élelmiszerekben nem fordul elő. 2 db pantetein molekula képez 1 db pantetin molekulát. A pantetein egy élelmiszerekben is előforduló B5-vitamin forma. A pantetin a lenyelés után a vékonybelünkben a felszívódása előtt panteteinné, majd sima pantoténsavvá (pantotenáttá) bomlik. [1,3,4,5,6] Részben már a vékonybélben, az ott bőségesen jelenlévő panteteináz enzim által lebomlik pantoténsavvá, míg egy része panteteinként vevődhet ugyan fel a bélsejtek által, amennyiben nagy a dózis, és a bél lumenben nincs elég enzim az átalakításához, azonban ez esetben a bélsejtekben bomlik le az általuk felvett pantetin pantoténsavvá. [5,6] A véráramba végül kizárólag pantoténsav formára bomolva kerül be a pantetin is. Ezt emberekben is és állatokban is vizsgálták. Embereknél egészen extrém dózisban adagolva a pantetint (1000 mg /kg) sem sikerült a véráramba akár csak nyomokban eljuttatni, csakis pantoténsavvá alakulva jelent meg a véráramban. [1,4] A pantetin és a Ca-pantotenát hasznosulása egy másik vizsgálatban össze is van hasonlítva. [6] A pantetinnek maga a felszívódása egy picit ugyan jobbnak tűnik, azonban gyorsabban ürült, így végeredményben a Ca-pantotenát dózisához képest 2x-es pantetin dózis is csak 18%-kal növelte jobban a B5 vérszintet, amiből azt állapították meg, hogy bár a pantetinnek is jó a hasznosulása, az elmarad a Ca-pantotenátétól, vagy maximum azonos. [6]
Mindebből egyértelműen következik, hogy a pantetin orálisan használva nem jelenthet előnyt a sima pantoténsavhoz képest, csak hátrányt a CoA szint növelésében, azaz a B5-vitamin hatását tekintve. Ennek ellenére mégis volt humán klinikai vizsgálat, ahol a pantetin nagy dózisának szájon át való szedését tették próbára, hátha hatásos lesz a CoA szint növelésére ún. PANK mutációval rendelkező gyerekek esetén. [2] Ez egy olyan genetikai probléma, ahol a pantoténsavat nem tudják a sejtek átalakítani az aktív CoA-vá, egymillióból egy-két embert érint, és egy PKAN nevű idegrendszeri betegséget okoz. PANK mutációval rendelkező muslincák pantetinnel való kezelése áthidalta ezt a konverziós problémát, emiatt próbálták ki embereknél is. [2] Felteszem, egyszerűbb volt szájon át szedve alkalmazni, és ezért nem intravénásan alkalmazták, bár ez számomra elég meglepő, mert már a korábbi vizsgálatokból tudhatták volna, hogy szájon át szedve nincs előnye a sima Ca-panotenáthoz képest. Nem is volt:
A PANK defektussal rendelkező gyerekek fél éven át napi 60 mg /kg pantetint kaptak (igen masszív dózis, 60 kg-os felnőttre átszámolva ez ugyanis 3600 mg /nap). A várt CoA szint növekedés elmaradt, alacsonyabb lett a vizsgálat végére, mint a kezdetekor. Elképzelhető ugyan, hogy segített mérsékelni az állapotuk romlását, azaz a CoA szintjük csökkenésének ütemét talán fékezhette, no de azt a sima pantoténsav (pl.: Ca-pantotenát) is fékezte volna, hiszen nem nulla PANK enzim-defektus esetén sem csak a konverzió hatékonysága rossz.
A Vitaminipar könyvben megadott referencia [Zorzi 2013] annak alátámasztására, hogy a pantetin áthidalja a konverziós problémákat, és emiatt jobb, mint a pantoténsav, egy olyan szakcikk volt, amelyben csupán egy pár soros említés szerepel a pantetinről: pont a PANK-enzimdefektusos muslincás vizsgálatot említik, hogy ennek fényében egy érdekes alternatív kezelés lehet a pantetin PANK defektus estén, hiszen a muslincáknál az képes volt CoA-vá alakulni, így érdemes lenne ezt a témát embereken is megvizsgálni… Ennyit írtak benne. Nyilván Zorzi és társai sem arra gondoltak ezzel a cikkükben, hogy szájon át adva lehetne hatásos… Alighanem a pantetin valóban hatásos is lenne PANK mutáció esetén vagy bármilyen esetben, ahol a pantoténsav->CoA konverzió sérült, csupán nem orálisan kellene beadni, hanem intravénásan, vagy még inkább nanokolloid rendszer részeként (pl.: liposzómák), így ugyanis el lehetne juttatni a sejtekhez és egyben a sejtekbe is változatlan formában, pantetinként, ami aztán panteteinné bomlana a sejtekben, ahol viszont már nem tudna visszabomlani pantoténsavvá, mivel a sejtekben belül már nincs ehhez enzim [1], így csak „előrefelé”, a CoA irányába tudna alakulni, azaz így valóban több lépéssel előrébb lenne, mint a pantoténsav, és áthidalhatná a problémát embereknél is, nem csak muslincáknál, akiknek nincsenek emésztőenzimjeik, amelyek a felszívódás előtt visszabontanák a pantetin egészét pantoténsavvá. A PKAN-tól szenvedő gyerekek érdekében reméljük hát, hogy ezt is meg fogják vizsgálni mihamarabb…
Bár B5-vitamin forrásként (CoA növelésre), mint láthattuk, a pantetin nem jobb, sőt picit még rosszabb is, mint pl. a Ca-pantotenát, azonban más célra nagyon is használható vegyületnek tűnik, de csakis óriási, farmakológiai/szuprafiziológiás dózisokban szedve, azaz nagyjából 1000 mg-os nagyságrendben szedve naponta. Ilyen dózisban jó hatással van a koleszterin és triglicerid szintekre, és mindenre, amire a ciszteamin nevű (természetes) gyógyszerhatóanyag is. A pantetin gyakorlatilag egy elnyújtott felszívódású ciszteaminnak tekinthető, ugyanis a belőle felszabaduló pantetein pantoténsavra és a ciszteaminra bomlik. [1,4] A B5-vitamintól eltérő hatását a ciszteaminná bomlásának köszönheti. Bár a ciszteamin hatásosabb, mint a pantetin, de sokak számára a ciszteamin nehezen tolerálható mellékhatásokat okozó gyógyszerhatóanyag, miközben a pantetin jól tolerálható bárki számára, gyakorlatilag nincs mellékhatása. [4] Ciszteamin helyettesítésére (pl. cisztin metabolizmus rendellenessége esetén) vagy a koleszterinszint szabályozására a pantetin kiváló hatóanyag. B5-vitaminnak használva azonban csak 10 x drágább, mint a sokat vizsgált Ca-pantotenát, de semmivel nem jobb.
Összefoglalva: A pantetinra nem kellene B5-vitamin forrásként tekinteni, mert bár B5-vitamin forrás, ilyen célra használni (CoA szint fokozására) hajszálnyit rosszabb, mint a jól bevált Ca-pantotenát. A ciszteamin mellékhatásoktól mentes alternatívájaként érdemes tekinteni rá. Napi 1000 mg körüli dózisban alkalmazva vérzsír/koleszterin szabályozó hatással bír egyebek mellett. Alacsonyabb dózisban alkalmazva azonban csak B5-vitamin forrásként funkcionál, de arra picit kevésbé jó, és kb. 10 x drágább (a Ca-pantotenát eleve drága, azaz ha pantetinre van cserélve egy termékben, annak fogyasztói ára feleslegesen és nagymértékben megemelkedik).
Felhasznált szakirodalom
(A bennük lévő releváns szöveget is mutatjuk, akit érdekel és nem szeretné végig bogarászni őket)
1. Czumaj A, Szrok-Jurga S, Hebanowska A, Turyn J, Swierczynski J, Sledzinski T, Stelmanska E. The Pathophysiological Role of CoA. International Journal of Molecular Sciences. 2020; 21(23):9057. https://doi.org/10.3390/ijms21239057
2. Chang X, Zhang J, Jiang Y, Yao B, Wang J, Wu Y. Pilot trial on the efficacy and safety of pantethine in children with pantothenate kinase-associated neurodegeneration: a single-arm, open-label study. Orphanet J Rare Dis. 2020;15(1):248. Published 2020 Sep 14. doi:10.1186/s13023-020-01530-5
3. Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front Nutr. 2019;6:48. Published 2019 Apr 17. doi:10.3389/fnut.2019.00048
4. Wittwer CT, Gahl WA, Butler JD, Zatz M, Thoene JG. Metabolism of pantethine in cystinosis. J Clin Invest. 1985;76(4):1665-1672. doi:10.1172/JCI112152
5. National Institute of Health, Pantothenic acid Fact Sheet for Health Professionals
Link: https://ods.od.nih.gov/factsheets/PantothenicAcid-HealthProfessional/
6. Scientific Opinion of the Panel on Food Additives and Nutrient Sources added to Food (ANS) on a request from the Commission on pantethine as a source for pantothenic acid added as a nutritional substance in food supplements. The EFSA Journal (2008) 865, 1-20.
Link: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2008.865
